Organic Chemistry: Halogenation Reactions
by Vincenzo Galloro
Halogenation of Alkanes
The reaction of a halogen with an alkane in the presence of UV light or heat leads to the formation of an alkyl halide. This reaction occurs when one or more hydrogen atoms in an organic compound is replaced with a halogen, such as fluorine and chlorine. A halogenation reaction can also be considered a substitution reaction. Typically, a carbon-hydrogen bond is broken and a new carbon-halogen bond is formed.
Ref: chemlibretexts
The general formation of an alkyl halide through halogenation of an alkane with a diatomic halogen can be seen below:
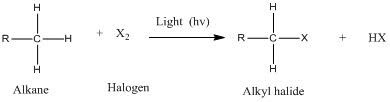
http://onlineorganicchemistrytutor.com/free-radicals-halogenation-of-alkane/
An example of halogenation between methane and chlorine can be seen below to produce chloromethane and hydrogen chloride:
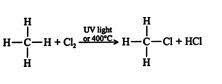
Halogenation of Alkenes
During halogenation of alkenes, the diatomic halogen reaction with the alkene by breaking the alkene’s double bond and replacing the hydrogens with halogens. More specifically, electrons in the double bond repel electrons in the diatomic halogen, causing polarization of the halogen bond. It should be noted that halogenation reactions of alkenes are also known as substitution reactions.
Ref: Chemlibre Text alkenes
An example of halogenation between ethene and diatomic chlorine can be seen below to produce 1,2-dichloroethane:

Halogenaton of Alkynes
The halogenation of an alkyne produces a halogenated alkene or, if excess halogen is present, an alkyl halide. The addition of a halogen to an alkyne proceeds in the same manner as a halogenation reaction with an alkene except the diatomic halogen breaks the triple bond of the alkyne to form a haloalkene. The reaction may proceed further and another halogenation reaction may take place, where the haloalkene is reacted with another diatomic halogen to produce a tetrahaloalkene.
The general formation of an haloalkene and tetrahaloalkane through halogenation of an alkyne and a diatomic halogen can be seen below:
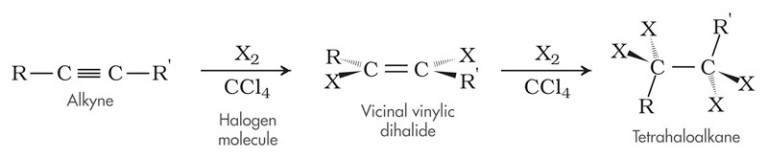
An example of halogenation between but-2-yne and bromine can be seen below to produce, 2,3-dibromobut-2-ene. The 2,3-dibromobut-2-ene undergoes halogenation once more with bromine to produce 2,2,3,3-tetrabromobutane:
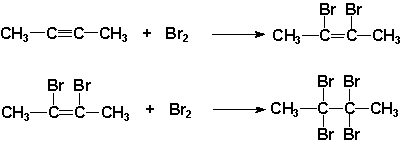
https://socratic.org/organic-chemistry-1/alkene-and-alkyne-addition-reactions/halogenation
